More General
In this section we will go over common Bismuth compounds, its atom model, and its properties.
Bismuth CompoundsBismuth Trihydride (BiH3)
Bismuth Triflouride (BiF3) Bismuth Pentaflouride (BiF5) Bismuth Trichloride (BiCl3) Bismuth Triiodine (BiI3) Dibismuth Trioxide (Bi2O2) Dibismuth Trisulphide (Bi2S3) Dibismuth Triselenide (Bi2Se3) Dibismuth Tritelluride (Bi2Te3) Bismuth Oxide (Bi2O3) Bismuth Subsalicylate (C7H5BiO4) (This is used in medication such as Pepto-Bismol) |
PartsOne part Bismuth, Three parts Hydrogen.
One part Bismuth, Three parts Flourine. One part Bismuth, Five parts Flourine. One part Bismuth, Three parts Chlorine. One part Bismuth, Three parts Iodine. Two parts Bismuth, Two parts Oxygen. Two parts Bismuth, Three Parts Sulfur. Two parts Bismuth, Three parts Selenium. Two parts Bismuth, Three parts Tellurium. Two parts Bismuth, Three parts Oxygen. Seven parts Carbon, Five parts Hydrogen, One part Bismuth, Four parts Oxygen. |
---------------------------------------------------------------------
Bismuth Atom Model
My Model |
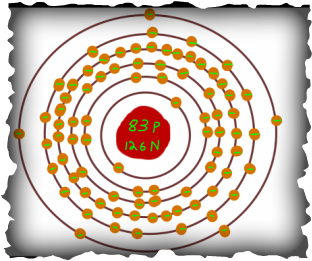
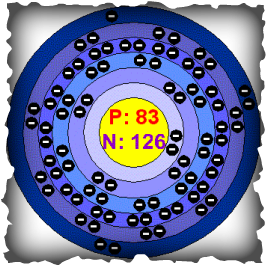
Since Bismuth is in period six, it has six rings. As shown with all three models, the nucleus has 83 protons and 126 neutrons. The first 5 rings of the atom are filled with electrons, 2, 8, 18, 32, and 20 respectively. The last ring has 5 electrons, or Bismuth's "Valance Electrons". These electrons help with Bismuth's chemical bonding with other elements.
|
---------------------------------------------------------------------
Bismuth's Properties
Physical
- Bismuth is a white crystalline metal. It is quite brittle and has a pinkish tinge to it.
- Bismuth has the second lowest thermal conductivity of all metals, second to only Mercury. Thermal conductivity is the amount of heat an element can hold and pass on to another element. - Bismuth is the most diamagnetic of all the metals. Diamagnetism is the ability to repel magnetic fields. - Bismuth has a high resistance to electric fields. - Boiling Point- 1837 K (1564 C, 2847 F) - Melting Point- 544.55 K (271.4 C, 520.52 F) - Density- 9.807 g/cm^3 |
Chemical
- All Bismuth salts, when placed in water, form compounds that aren't able to be dissolved.
- Bismuth dissolves in nitric air. - When heated in air, Bismuth burns. |